FDA and Merck have known about these harms for 25 years and yet failed to warn pediatricians or parents
Background and context
When Merck’s M-M-R II was licensed in 1978, a proper clinical trial of its safety was never conducted. From a prior ICAN demand to the FDA, we know that the safety was only assessed in 834 children for a period of 42 days in trials that had no placebo control. Further, within the first 42 days following administration of the vaccine, in one of the largest trials for this vaccine, 63% of the children experienced gastrointestinal illness and 42% had upper respiratory illness. Yet the FDA approved the product anyway.
Ignoring troubling safety signals appears to be a pattern with the FDA. Fast forward to 1995 – following licensure of another Merck vaccine, VARIVAX (attenuated, live, varicella, a.k.a. chickenpox, vaccine), the FDA required a post-licensure safety study which Merck conducted from June 1, 1995 through February 5, 1997. In 2019, ICAN, though its attorneys, filed a Freedom of Information Act request for that post-licensure study, which the FDA finally produced a few months ago.
The discovery of the VARIVAX post-licensure study
Merck’s Phase-IV post-licensure study included 34,655 children 12-23 months old and 51,463 children 2 to 12 years of age who were injected with VARIVAX. About 60% of these children aged 12-23 months and 17% of these children aged 2 to 12 years also received the improperly tested M-M-R II vaccine, in addition to other vaccines, at the same time they received VARIVAX. The study found several troubling safety signals.
The study found more than 60 conditions including allergic reactions, alopecia, arthritis/arthralgia, and gastroenteritis that were significantly elevated following VARIVAX vaccination. This suggests this vaccine, soon after administration, may lead to a wide range of reactions that in many cases are worse than the usual chickenpox rash.
But then there was this additional bombshell that, as far as we know, has never been disclosed to the public. The post-licensure study shows that there are significantly more adverse events among children receiving both VARIVAX and M-M-R II compared to those receiving VARIVAX alone (summary table below).
Every parent should be horrified by this information. To be clear: what is notable here is that the FDA knew, as early as the first report of this study in September 1997, that the likelihood of an adverse event in children 12-23 months old and 2-12 years old appears significantly increased if the child receives VARIVAX at the same time as the M-M-R II vaccine.
The study also noted: “It should be born in mind that concomitant vaccination with MMR could be a marker for vaccination with several other vaccines simultaneously.” This raises multiple red flags. The FDA and Merck know that VARIVAX is linked with increased rates of troubling adverse events. And the FDA and Merck know that the CDC schedule increases the rate of harms given that it calls for concomitant administration of VARIVAX and MMR (and potentially “several other vaccines”)!
So, what did the FDA do about it? Did it reevaluate the licensure of VARIVAX? No. Did it reevaluate the licensure of MMR, M-M-R II, or MMRV? No. Did it, at the very least, immediately recommend that VARIVAX and M-M-R II no longer be given concomitantly? No. And it still has not done so, 25 years later. The FDA had a legal and moral obligation to release this information to the public so that parents could make informed decisions. Of course, as we have seen repeatedly, the FDA is loath to admit it has made a mistake. So, this report was essentially buried for 25 years until ICAN finally took action to obtain a copy.
More than two decades later, the FDA has not even bothered to update the package inserts for M-M-R II to disclose these harms! In fact, despite the results of this study, the package insert (See below) for VARIVAX today explicitly claims that administering the two together is perfectly safe: “VARIVAX may be administered concomitantly with M-M-R II.”
This is yet another example of deception on the American people and why federal health authorities should have no role in recommending, let alone mandating, any product, but especially any product that can have adverse effects. Once federal health authorities become partners in the success of a vaccine product, including having their reputations tied to its success and being the party responsible for defending against claims it causes harm, admitting issues or mistakes becomes a devastating proposition to the very same health authorities that are supposed to ensure vaccine safety. This conflict is intractable and destructive. ICAN will never stop fighting to bring you the truth and making sure that every last government official that has failed to safeguard the health of our children is held accountable.
ICAN OBTAINS COPIES OF CLINICAL TRIAL RELIED UPON TO LICENSE MMR VACCINE WHICH REVEAL THAT IT SHOULD NEVER HAVE BEEN LICENSED
Merck sells the only measles, mumps, and rubella vaccine in the United States, trade named M-M-R-II (MMR-II). MMR-II is produced by growing viruses on biological medium, including human diploid lung fibroblasts from an aborted fetus, and was licensed by the FDA in 1978 for people aged 12 months and older.
Prior to FDA licensure of a new experimental vaccines, such as MMR-II, they are expected to undergo long-term placebo-controlled clinical trials with typically tens of thousands of participants to assure their safety.
To evaluate whether Merck met any of these criteria, ICAN, through its attorneys, demanded on August 20, 2018 that the FDA produce copies of all the clinical trial reports relied upon to license Merck’s MMR-II vaccine. The FDA eventually, on March 27, 2019 produced to ICAN copies of the clinical trial reports for this vaccine, totaling 215 pages. What do these documents show? They show that this product should never have been licensed.
It should not have been licensed because MMR-II was licensed by the FDA based on clinical trials which had a total of 834 children, had no placebo control, and only reviewed safety for 42 days after injection! Putting aside the lack of placebo control, even if the clinical trials were properly controlled, they did not have enough individuals to assess safety; nor did they review safety for long enough.
Despite the fact that approximately a third of the children in the clinical trials developed gastrointestinal issues and respiratory issues within 42 days of receiving MMR-II, due to their underpowered size and lack of follow-up, they were able to avoid this being a roadblock to licensure. Despite this vaccine being licensed, the clinical trials relied upon to license this vaccine clearly did not, as they could not, confirm that the product was safe, and certainly not for any period longer than 42 days, nor for even the 42 days they did review safety.
For example, the below table is the safety data from one of the largest clinical trials for this vaccine, which had a total of just 102 children injected with MMR-II:
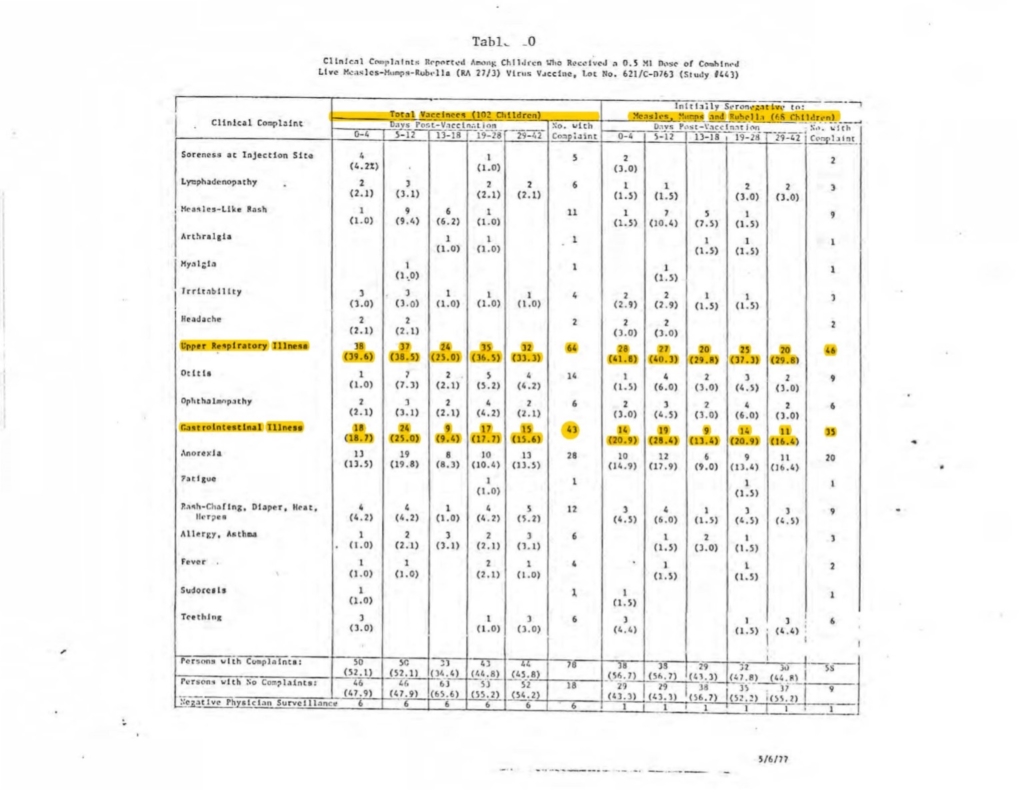
The table above shows that of 102 children injected with MMR, 64 of them, or nearly 63%, experienced gastrointestinal illness and that 43, or 42%, of the children experienced upper respiratory illness within the first 42 days following administration. That differential was apparently deemed acceptable by the FDA because Merck’s paid investigator presumably did not consider these serious adverse events to be related to MMR-II.
After the current MMR’s licensure in 1978, its use in children steadily increased and lawsuits from injuries from this product also began to snowball. Indeed, by the mid-1980s – when the only two commonly injected childhood vaccines were MMR and DTP – pharmaceutical companies were facing crippling liability from their vaccine products due to lawsuits brought by parents whose children were injured by these products. As the United States Supreme Court explained: “by the mid-1980’s … the remaining [vaccine] manufacturer estimated that its potential tort liability exceeded its annual sales by a factor of 200.”
Instead of letting the usual market forces drive pharmaceutical companies to develop safer vaccines, Congress passed the National Childhood Vaccine Injury Act in 1986, which virtually eliminated economic liability for pharmaceutical companies for injuries caused by their vaccine products. While Merk has paid billions of dollars for misconduct and injuries related to their drug products, it cannot be held accountable for misconduct and injuries related to MMR-II.
Once licensed and widely promoted by our federal and state health authorities, it should not be surprising that they do not want to admit to the numerous safety issues with this product that have arisen after licensure. And it is clear there are many safety issues. After licensure, federal law expressly provides that Merck shall include in the package insert for MMR-II “only those adverse events for which there is some basis to believe there is a causal relationship between the drug and the occurrence of the adverse event.” The package insert for MMR-II lists dozens of such adverse reactions that Merck has identified, many of which are serious and debilitating.
The CDC also discloses that MMR vaccine can cause deafness, long term seizure, coma, and brain damage. An example of such an injury involved a $100 million award to the victim of an MMR injury. This high rate of hospitalization and emergency room visits from MMR vaccine is confirmed in a study conducted by Canadian health authorities of 271,495 children after their 12-month MMR. This study set out to confirm the safety of MMR, but what they found instead was that “[t]here was a significantly elevated risk of primary emergency room visits approximately one to two weeks following 12- and 18-month vaccination.” This amounted to an additional “one event for every 158 vaccinated” children receiving MMR. Extrapolating these figures to the United States, 63,291 additional American children visit the emergency room each year because of the MMR program.
It is also worth pointing out that the first vaccine for measles was licensed in the United States in 1963 and, according to the CDC, the mortality rate from measles declined by over 98% between 1900 and 1962. In the years leading up to 1963 (when no measles vaccine existed), the CDC reported a total of approximately 400 deaths from measles per year in the United States during a time when virtually every American had measles, reflecting an annual death rate from measles of 1 in 500,000 Americans prior to the introduction of the measles vaccine.
Eliminating measles has demonstrably and measurably increased certain cancer rates, the risk of heart disease, and other serious medical conditions:
- The International Agency for Research on Cancer has confirmed that those who never had measles had a 66% increased rate of Non-Hodgkin Lymphoma and a 233% increased rate of Hodgkin’s Lymphoma. These two cancers killed 20,960 Americans in 2018.
- Likewise, researchers at the Department of Health Care and Epidemiology at the University of British Columbia and the Department of Biology at the University of Victoria have confirmed that those who never had measles had a 50% increased rate of ovarian cancer, which killed 14,070 Americans in 2018.
- The nation of Japan concluded, after tracking over 100,000 of its citizens for more than 22 years, that having measles and mumps was “associated with lower risks of mortality from heart disease,” which killed 655,000Americans each year.
- Additionally, studies reflect that children who have had measles have far less allergies and atopic diseases, such as asthma, and adults who had measles have a reduced risk of Parkinson’s Disease.
Hence, the evidence shows that eliminating measles has caused far more deaths annually in the United States from cancer and heart disease than the potentially few hundred lives saved from elimination of measles.
In any event, the FDA’s basis for licensing MMR-II is incredible when considering that: (i) states mandate by law that millions of children receive this vaccine every year; (ii) Merck cannot be sued for most injuries caused by this product under federal law; and (iii) Merck’s sales of this product, alone or in combination with another of its products, is in the billions of dollars. But yet, the FDA licensed this product based on a clinical trial with no placebo control, with less than 1,000 children, and that reviewed safety for only 42 days!
